what mass of potassium chlorate is required to supply the proper amount of oxygen
Affiliate four. Stoichiometry of Chemical Reactions
4.ane Writing and Balancing Chemical Equations
Learning Objectives
By the end of this section, you will exist able to:
- Derive chemical equations from narrative descriptions of chemical reactions.
- Write and balance chemic equations in molecular, total ionic, and net ionic formats.
The preceding chapter introduced the employ of element symbols to represent individual atoms. When atoms gain or lose electrons to yield ions, or combine with other atoms to form molecules, their symbols are modified or combined to generate chemical formulas that accordingly represent these species. Extending this symbolism to stand for both the identities and the relative quantities of substances undergoing a chemical (or physical) change involves writing and balancing a chemical equation. Consider equally an example the reaction betwixt one marsh gas molecule (CHiv) and 2 diatomic oxygen molecules (O2) to produce one carbon dioxide molecule (CO2) and 2 water molecules (HtwoO). The chemic equation representing this process is provided in the upper half of Effigy i, with space-filling molecular models shown in the lower half of the effigy.
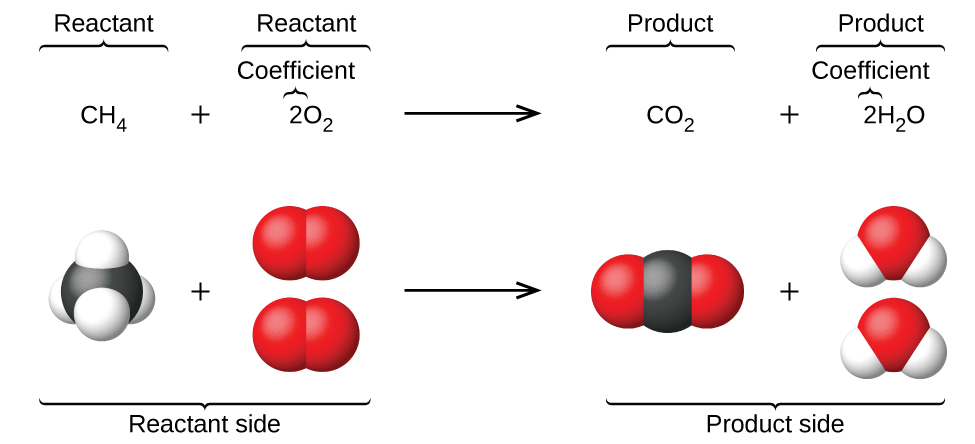
This example illustrates the central aspects of whatever chemical equation:
- The substances undergoing reaction are called reactants, and their formulas are placed on the left side of the equation.
- The substances generated by the reaction are called products, and their formulas are placed on the right sight of the equation.
- Plus signs (+) separate private reactant and production formulas, and an arrow (⟶) separates the reactant and production (left and right) sides of the equation.
- The relative numbers of reactant and product species are represented by coefficients (numbers placed immediately to the left of each formula). A coefficient of one is typically omitted.
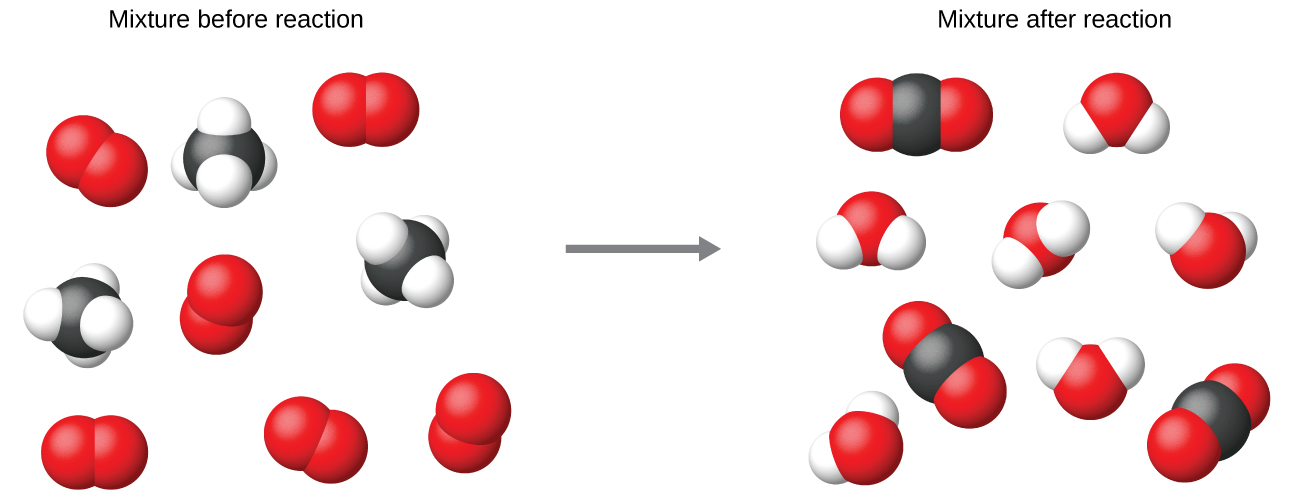
It is mutual do to utilise the smallest possible whole-number coefficients in a chemical equation, as is done in this example. Realize, all the same, that these coefficients stand for the relative numbers of reactants and products, and, therefore, they may be correctly interpreted as ratios. Methane and oxygen react to yield carbon dioxide and h2o in a ane:2:1:2 ratio. This ratio is satisfied if the numbers of these molecules are, respectively, ane-2-1-2, or ii-four-2-4, or 3-6-3-6, and so on (Figure 2). As well, these coefficients may be interpreted with regard to whatsoever amount (number) unit of measurement, and so this equation may be correctly read in many means, including:
- I methane molecule and two oxygen molecules react to yield one carbon dioxide molecule and two water molecules.
- One dozen marsh gas molecules and 2 dozen oxygen molecules react to yield one dozen carbon dioxide molecules and 2 dozen water molecules.
- One mole of methane molecules and two moles of oxygen molecules react to yield one mole of carbon dioxide molecules and 2 moles of water molecules.
Balancing Equations
The chemical equation described in section four.1 is balanced, significant that equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. This is a requirement the equation must satisfy to exist consistent with the law of conservation of affair. It may be confirmed past simply summing the numbers of atoms on either side of the pointer and comparison these sums to ensure they are equal. Notation that the number of atoms for a given chemical element is calculated by multiplying the coefficient of any formula containing that element by the chemical element's subscript in the formula. If an element appears in more than one formula on a given side of the equation, the number of atoms represented in each must be computed and so added together. For example, both product species in the example reaction, COtwo and H2O, contain the element oxygen, and so the number of oxygen atoms on the product side of the equation is
[latex](one \;\text{CO}_2 \;\text{molecule} \times \frac{ii \;\text{O atoms}}{\text{CO}_2 \;\text{molecule}}) + (2\;\text{H}_2\text{O molecule} \times \frac{i \;\text{O cantlet}}{\text{H}_2\text{O molecule}}) = iv \;\text{O atoms}[/latex]
The equation for the reaction betwixt methyl hydride and oxygen to yield carbon dioxide and water is confirmed to be balanced per this approach, equally shown here:
[latex]\text{CH}_4 + 2\text{O}_2 \longrightarrow \text{CO}_2 + 2\text{H}_2\text{O}[/latex]
| Element | Reactants | Products | Balanced? |
|---|---|---|---|
| C | one × 1 = 1 | 1 × ane = one | 1 = i, yep |
| H | 4 × 1 = 4 | 2 × two = 4 | four = 4, yes |
| O | 2 × 2 = 4 | (one × 2) + (2 × 1) = 4 | 4 = 4, yes |
| Table 1. | |||
A balanced chemical equation frequently may be derived from a qualitative description of some chemical reaction by a adequately simple approach known every bit balancing by inspection. Consider as an example the decomposition of water to yield molecular hydrogen and oxygen. This process is represented qualitatively by an unbalanced chemic equation:
[latex]\text{H}_2\text{O} \longrightarrow \text{H}_2 + \text{O}_2 \;(\text{unbalanced})[/latex]
Comparing the number of H and O atoms on either side of this equation confirms its imbalance:
| Element | Reactants | Products | Balanced? |
|---|---|---|---|
| H | one × two = two | ane × 2 = 2 | 2 = 2, yes |
| O | 1 × 1 = 1 | 1 × 2 = two | 1 ≠ 2, no |
| Table ii. | |||
The numbers of H atoms on the reactant and product sides of the equation are equal, merely the numbers of O atoms are not. To accomplish rest, the coefficients of the equation may be changed as needed. Go along in mind, of class, that the formula subscripts ascertain, in part, the identity of the substance, and and then these cannot exist changed without altering the qualitative meaning of the equation. For example, irresolute the reactant formula from HtwoO to HtwoO2 would yield remainder in the number of atoms, but doing so also changes the reactant's identity (it's at present hydrogen peroxide and non water). The O atom residuum may be achieved past changing the coefficient for H2O to 2.
[latex]2\text{H}_2\text{O} \longrightarrow \text{H}_2 + \text{O}_2 \;(\text{unbalanced})[/latex]
| Element | Reactants | Products | Balanced? |
|---|---|---|---|
| H | ii × two = 4 | i × ii = two | iv ≠ 2, no |
| O | two × 1 = two | i × 2 = 2 | 2 = two, yeah |
| Table 3. | |||
The H atom balance was upset by this alter, but it is easily reestablished by changing the coefficient for the H2 product to 2.
[latex]two\text{H}_2\text{O} \longrightarrow 2\text{H}_2 + \text{O}_2 \;(\text{balanced})[/latex]
| Element | Reactants | Products | Balanced? |
|---|---|---|---|
| H | 2 × two = iv | 2 × ii = 4 | four = 4, yeah |
| O | 2 × 1 = two | 1 × two = 2 | 2 = 2, yes |
| Table 4. | |||
These coefficients yield equal numbers of both H and O atoms on the reactant and product sides, and the counterbalanced equation is, therefore:
[latex]2\text{H}_2\text{O} \longrightarrow 2\text{H}_2 + \text{O}_2[/latex]
Example 1
Balancing Chemical Equations
Write a balanced equation for the reaction of molecular nitrogen (Northward2) and oxygen (Oii) to course dinitrogen pentoxide.
Solution
Beginning, write the unbalanced equation.
[latex]\text{N}_2 + \text{O}_2 \longrightarrow \text{N}_2 \text{O}_5 \;(\text{unbalanced})[/latex]
Side by side, count the number of each type of atom present in the unbalanced equation.
| Chemical element | Reactants | Products | Balanced? |
|---|---|---|---|
| N | 1 × ii = ii | one × 2 = 2 | 2 = 2, yes |
| O | i × 2 = 2 | one × 5 = five | 2 ≠ five, no |
| Table v. | |||
Though nitrogen is balanced, changes in coefficients are needed to residue the number of oxygen atoms. To balance the number of oxygen atoms, a reasonable kickoff attempt would exist to change the coefficients for the Otwo and N2O5 to integers that volition yield 10 O atoms (the least mutual multiple for the O atom subscripts in these two formulas).
[latex]\text{North}_2 + 5\text{O}_2 \longrightarrow 2\text{N}_2\text{O}_5 \;(\text{unbalanced})[/latex]
| Chemical element | Reactants | Products | Balanced? |
|---|---|---|---|
| Due north | 1 ×× 2 = ii | 2 × ii = 4 | ii ≠ four, no |
| O | five × 2 = 10 | 2 × 5 = ten | 10 = ten, yes |
| Table 6. | |||
The N cantlet residue has been upset past this change; information technology is restored by changing the coefficient for the reactant Nii to 2.
[latex]2\text{N}_2 + 5\text{O}_2 \longrightarrow 2\text{N}_2 \text{O}_5[/latex]
| Element | Reactants | Products | Counterbalanced? |
|---|---|---|---|
| N | 2 × 2 = 4 | 2 × ii = 4 | 4 = 4, yes |
| O | v × ii = ten | 2 × 5 = ten | 10 = 10, yes |
| Table 7. | |||
The numbers of Northward and O atoms on either side of the equation are now equal, and so the equation is balanced.
Check Your Learning
Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and water. (Hint: Residue oxygen last, since it is present in more than 1 molecule on the right side of the equation.)
Answer:
[latex]2\text{NH}_4 \text{NO}_3 \longrightarrow ii\text{Due north}_2 + \text{O}_2 + 4\text{H}_2\text{O}[/latex]
It is sometimes convenient to use fractions instead of integers as intermediate coefficients in the process of balancing a chemical equation. When balance is achieved, all the equation's coefficients may so be multiplied by a whole number to convert the fractional coefficients to integers without upsetting the atom balance. For instance, consider the reaction of ethane (C2H6) with oxygen to yield HiiO and CO2, represented by the unbalanced equation:
[latex]\text{C}_2 \text{H}_6 + \text{O}_2 \longrightarrow \text{H}_2 \text{O} + \text{C} \text{O}_2 \;(\text{unbalanced})[/latex]
Post-obit the usual inspection approach, ane might offset balance C and H atoms by changing the coefficients for the two product species, as shown:
[latex]\text{C}_2 \text{H}_6 + \text{O}_2 \longrightarrow three\text{H}_2 \text{O} + 2\text{C} \text{O}_2 \;(\text{unbalanced})[/latex]
This results in seven O atoms on the product side of the equation, an odd number—no integer coefficient can be used with the Otwo reactant to yield an odd number, so a fractional coefficient, [latex]\frac{7}{2}[/latex], is used instead to yield a provisional balanced equation:
[latex]\text{C}_2 \text{H}_6 + \frac{vii}{2}\text{O}_2 \longrightarrow 3\text{H}_2 \text{O} + 2\text{C} \text{O}_2 \;[/latex]
A conventional balanced equation with integer-only coefficients is derived by multiplying each coefficient by ii:
[latex]ii\text{C}_2 \text{H}_6 + 7\text{O}_2 \longrightarrow 6\text{H}_2 \text{O} + 4\text{C} \text{O}_2 \;[/latex]
Finally with regard to balanced equations, recall that convention dictates use of the smallest whole-number coefficients. Although the equation for the reaction between molecular nitrogen and molecular hydrogen to produce ammonia is, indeed, balanced,
[latex]3\text{N}_2 + 9\text{H}_2 \longrightarrow 6\text{N} \text{H}_3[/latex]
the coefficients are not the smallest possible integers representing the relative numbers of reactant and product molecules. Dividing each coefficient by the greatest common gene, three, gives the preferred equation:
[latex]\text{North}_2 + 3\text{H}_2 \longrightarrow ii\text{N} \text{H}_3[/latex]

Use this interactive tutorial for boosted practice balancing equations.
Additional Information in Chemical Equations
The physical states of reactants and products in chemical equations very often are indicated with a parenthetical abbreviation following the formulas. Common abbreviations include due south for solids, l for liquids, thou for gases, and aq for substances dissolved in h2o (aqueous solutions, as introduced in the preceding affiliate). These notations are illustrated in the example equation here:
[latex]2\text{Na}(southward) + 2\text{H}_2 \text{O}(fifty) \longrightarrow ii\text{NaOH}(aq) + \text{H}_2(thou)[/latex]
This equation represents the reaction that takes place when sodium metal is placed in h2o. The solid sodium reacts with liquid water to produce molecular hydrogen gas and the ionic compound sodium hydroxide (a solid in pure course, but readily dissolved in water).
Special conditions necessary for a reaction are sometimes designated past writing a discussion or symbol above or below the equation'south arrow. For example, a reaction carried out by heating may be indicated past the uppercase Greek letter delta (Δ) over the arrow.
[latex]\text{CaCO}_3(s) \;\xrightarrow{\Delta} \; \text{CaO}(s) + \text{CO}_2(yard)[/latex]
Other examples of these special conditions volition be encountered in more depth in afterward chapters.
Equations for Ionic Reactions
Given the abundance of h2o on earth, it stands to reason that a great many chemical reactions take place in aqueous media. When ions are involved in these reactions, the chemical equations may be written with diverse levels of detail appropriate to their intended use. To illustrate this, consider a reaction between ionic compounds taking place in an aqueous solution. When aqueous solutions of CaCltwo and AgNO3 are mixed, a reaction takes place producing aqueous Ca(NOiii)ii and solid AgCl:
[latex]\text{CaCl}_2(aq) + two\text{AgNO}_3(aq) \longrightarrow \text{Ca(NO}_3)_2(aq) + 2\text{AgCl}(due south)[/latex]
This balanced equation, derived in the usual fashion, is called a molecular equation because it doesn't explicitly represent the ionic species that are nowadays in solution. When ionic compounds deliquesce in h2o, they may dissociate into their constituent ions, which are subsequently dispersed homogenously throughout the resulting solution (a thorough give-and-take of this important procedure is provided in the chapter on solutions). Ionic compounds dissolved in h2o are, therefore, more realistically represented equally dissociated ions, in this case:
[latex]\begin{assortment}{r @{{}\longrightarrow{}} l} \text{CaCl}_2(aq) & \text{Ca}^{2+}(aq) + 2 \text{Cl}^{-}(aq) \\[0.5em] 2 \text{AgNO}_3(aq) & ii\text{Ag}^{+}(aq) + 2 {\text{NO}_3}^{-}(aq) \\[0.5em] \text{Ca(NO}_3)_2(aq) & \text{Ca}^{2+}(aq) + 2 {\text{NO}_3}^{-}(aq) \stop{assortment}[/latex]
Unlike these three ionic compounds, AgCl does not dissolve in water to a significant extent, every bit signified by its concrete state notation, s.
Explicitly representing all dissolved ions results in a complete ionic equation. In this item case, the formulas for the dissolved ionic compounds are replaced by formulas for their dissociated ions:
[latex]\text{Ca}^{two+}(aq) + 2\text{Cl}^{-}(aq) + 2\text{Ag}^{+}(aq) + 2{\text{NO}_3}^{-}(aq) \longrightarrow \text{Ca}^{two+}(aq) + ii{\text{NO}_3}^{-}(aq) + 2\text{AgCl}(due south)[/latex]
Examining this equation shows that ii chemical species are present in identical form on both sides of the arrow, Ca2+(aq) and NO3−(aq).NO3−(aq). These spectator ions—ions whose presence is required to maintain charge neutrality—are neither chemically nor physically inverse by the process, and so they may be eliminated from the equation to yield a more succinct representation chosen a internet ionic equation:
[latex]\rule[0.5ex]{4em}{0.1ex}\hspace{-4em} \text{Ca}^{2+}(aq) + 2\text{Cl}^{-}(aq) + 2\text{Ag}^{+}(aq) + \rule[0.5ex]{4.5em}{0.1ex}\hspace{-4.5em} 2\text{NO}_3^{-}(aq) \longrightarrow \rule[0.5ex]{4em}{0.1ex}\hspace{-4em} \text{Ca}^{2+}(aq) + \rule[0.5ex]{4.5em}{0.1ex}\hspace{-four.5em} ii{\text{NO}_3}^{-}(aq) + two\text{AgCl}(southward)[/latex]
[latex]2\text{Cl}^{-}(aq) + 2\text{Ag}^{+}(aq) \longrightarrow 2\text{AgCl}(s)[/latex]
Following the convention of using the smallest possible integers as coefficients, this equation is then written:
[latex]\text{Cl}^{-}(aq) + \text{Ag}^{+}(aq) \longrightarrow \text{AgCl}(south)[/latex]
This net ionic equation indicates that solid silver chloride may be produced from dissolved chloride and silver(I) ions, regardless of the source of these ions. These molecular and consummate ionic equations provide additional information, namely, the ionic compounds used equally sources of Cl− and Ag+.
Case 2
Molecular and Ionic Equations
When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid h2o. Write balanced molecular, complete ionic, and internet ionic equations for this process.
Solution
Begin by identifying formulas for the reactants and products and arranging them properly in chemical equation course:
[latex]\text{CO}_2(aq) + \text{NaOH}(aq) \longrightarrow \text{Na}_2 \text{CO}_3(aq) + \text{H}_2 \text{O}(l) \;(\text{unbalanced})[/latex]
Residue is achieved hands in this case by changing the coefficient for NaOH to two, resulting in the molecular equation for this reaction:
[latex]\text{CO}_2(aq) + 2\text{NaOH}(aq) \longrightarrow \text{Na}_2 \text{CO}_3(aq) + \text{H}_2 \text{O}(fifty)[/latex]
The two dissolved ionic compounds, NaOH and NaiiCO3, tin be represented as dissociated ions to yield the consummate ionic equation:
[latex]\text{CO}_2(aq) + 2\text{Na}^{+}(aq) + 2\text{OH}^{-}(aq) \longrightarrow 2\text{Na}^{+}(aq) + {\text{CO}_3}^{ii-}(aq) + \text{H}_2 \text{O}(l)[/latex]
Finally, identify the spectator ion(s), in this case Na+(aq), and remove it from each side of the equation to generate the net ionic equation:
[latex]\text{CO}_2(aq) + \dominion[0.5ex]{4.25em}{0.1ex}\hspace{-4.25em} two\text{Na}^{+}(aq) + 2\text{OH}^{-}(aq) \longrightarrow \rule[0.5ex]{4.25em}{0.1ex}\hspace{-iv.25em} 2\text{Na}^{+}(aq) + {\text{CO}_3}^{two-}(aq) + \text{H}_2 \text{O}(fifty)[/latex][latex]\text{CO}_2(aq) + 2\text{OH}^{-}(aq) \longrightarrow {\text{CO}_3}^{two-}(aq) + \text{H}_2 \text{O}(l)[/latex]
Check Your Learning
Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via the electrolysis of brine, according to the following unbalanced equation:
[latex]\text{NaCl}(aq) + \text{H}_2 \text{O}(l) \;\;\xrightarrow{\text{electricity}}\;\; \text{NaOH}(aq) + \text{H}_2(g) + \text{Cl}_2(g)[/latex]
Write balanced molecular, complete ionic, and net ionic equations for this process.
Answer:
[latex]2\text{NaCl}(aq) + 2\text{H}_2 \text{O} \longrightarrow ii \text{NaOH}(aq) + \text{H}_2(g) + \text{Cl}_2(g) (\text{molecular})[/latex]
[latex]2\text{Na}^{+}(aq) + 2\text{Cl}^{-}(aq) + 2\text{H}_2 \text{O} \longrightarrow 2\text{Na}^{+}(aq) + ii\text{OH}^{-}(aq) + \text{H}_2(g) + \text{Cl}_2(k) (\text{complete ionic})[/latex]
[latex]2\text{Cl}^{-}(aq) + two\text{H}_2 \text{O} \longrightarrow 2\text{OH}^{-}(aq) + 2\text{H}_2(g) + \text{Cl}_2(g) (\text{cyberspace ionic})[/latex]
Key Concepts and Summary
Chemical equations are symbolic representations of chemic and concrete changes. Formulas for the substances undergoing the modify (reactants) and substances generated past the alter (products) are separated by an arrow and preceded by integer coefficients indicating their relative numbers. Balanced equations are those whose coefficients result in equal numbers of atoms for each element in the reactants and products. Chemical reactions in aqueous solution that involve ionic reactants or products may be represented more realistically past complete ionic equations and, more succinctly, past internet ionic equations.
Chemistry Stop of Chapter Exercises
- What does it mean to say an equation is balanced? Why is it important for an equation to be counterbalanced?
- Consider molecular, complete ionic, and net ionic equations.
(a) What is the deviation betwixt these types of equations?
(b) In what circumstance would the consummate and net ionic equations for a reaction exist identical?
- Residuum the following equations:
(a) [latex]\text{PCl}_5(s) + \text{H}_2 \text{O}(50) \longrightarrow \text{POCl}_3(l) + \text{HCl}(aq)[/latex]
(b) [latex]\text{Cu}(s) + \text{HNO}_3(aq) \longrightarrow \text{Cu(NO}_3)_2(aq) + \text{H}_2 \text{O}(l) + \text{NO}(g)[/latex]
(c) [latex]\text{H}_2(g) + \text{I}_2(due south) \longrightarrow \text{Hullo}(s)[/latex]
(d) [latex]\text{Fe}(s) + \text{O}_2(g) \longrightarrow \text{Fe}_2 \text{O}_3(due south)[/latex]
(e) [latex]\text{Na}(s) + \text{H}_2 \text{O}(l) \longrightarrow \text{NaOH}(aq) + \text{H}_2(g)[/latex]
(f) [latex]\text{(NH}_4)_2 \text{Cr}_2\text{O}_7(s) \longrightarrow \text{Cr}_2\text{O}_3(south) + \text{N}_2(g) + \text{H}_2 \text{O}(one thousand)[/latex]
(yard) [latex]\text{P}_4(s) + \text{Cl}_2(g) \longrightarrow \text{PCl}_3(l)[/latex]
(h) [latex]\text{PtCl}_4(due south) \longrightarrow \text{Pt}(s) + \text{Cl}_2(chiliad)[/latex]
- Remainder the following equations:
(a) [latex]\text{Ag}(due south) + \text{H}_2 \text{S}(g) + \text{O}_2(g) \longrightarrow \text{Ag}_2 \text{S}(southward) + \text{H}_2 \text{O}(l)[/latex]
(b) [latex]\text{P}_4(s) + \text{O}_2(g) \longrightarrow \text{P}_4 \text{O}_{10}(s)[/latex]
(c) [latex]\text{Pb}(south) + \text{H}_2 \text{O}(l) + \text{O}_2(g) \longrightarrow \text{Pb(OH)}_2(s)[/latex]
(d) [latex]\text{Fe}(south) + \text{H}_2 \text{O}(l) \longrightarrow \text{Atomic number 26}_3 \text{O}_4(s) + \text{H}_2(g)[/latex]
(east) [latex]\text{Sc}_2 \text{O}_3(s) + \text{SO}_3(l) \longrightarrow \text{Sc}_2 \text{(And so}_4)_3(southward)[/latex]
(f) [latex]\text{Ca}_3 \text{(PO}_4)_2(aq) + \text{H}_3 \text{PO}_4(aq) \longrightarrow \text{Ca(H}_2 \text{PO}_4)_2(aq)[/latex]
(chiliad) [latex]\text{Al}(south) + \text{H}_2 \text{So}_4(aq) \longrightarrow \text{Al}_2 \text{(And so}_4)_3(s) + \text{H}_2(chiliad)[/latex]
(h) [latex]\text{TiCl}_4(southward) + \text{H}_2 \text{O}(g) \longrightarrow \text{TiO}_2(s) + \text{HCl}(thou)[/latex]
- Write a balanced molecular equation describing each of the following chemical reactions.
(a) Solid calcium carbonate is heated and decomposes to solid calcium oxide and carbon dioxide gas.
(b) Gaseous butane, CfourH10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and h2o vapor.
(c) Aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride.
(d) Water vapor reacts with sodium metal to produce solid sodium hydroxide and hydrogen gas.
- Write a counterbalanced equation describing each of the post-obit chemical reactions.
(a) Solid potassium chlorate, KClO3, decomposes to form solid potassium chloride and diatomic oxygen gas.
(b) Solid aluminum metal reacts with solid diatomic iodine to form solid Al2I6.
(c) When solid sodium chloride is added to aqueous sulfuric acid, hydrogen chloride gas and aqueous sodium sulfate are produced.
(d) Aqueous solutions of phosphoric acid and potassium hydroxide react to produce aqueous potassium dihydrogen phosphate and liquid water.
- Colorful fireworks oft involve the decomposition of barium nitrate and potassium chlorate and the reaction of the metals magnesium, aluminum, and iron with oxygen.
(a) Write the formulas of barium nitrate and potassium chlorate.
(b) The decomposition of solid potassium chlorate leads to the formation of solid potassium chloride and diatomic oxygen gas. Write an equation for the reaction.
(c) The decomposition of solid barium nitrate leads to the formation of solid barium oxide, diatomic nitrogen gas, and diatomic oxygen gas. Write an equation for the reaction.
(d) Write separate equations for the reactions of the solid metals magnesium, aluminum, and iron with diatomic oxygen gas to yield the respective metal oxides. (Assume the iron oxide contains Iron3+ ions.)
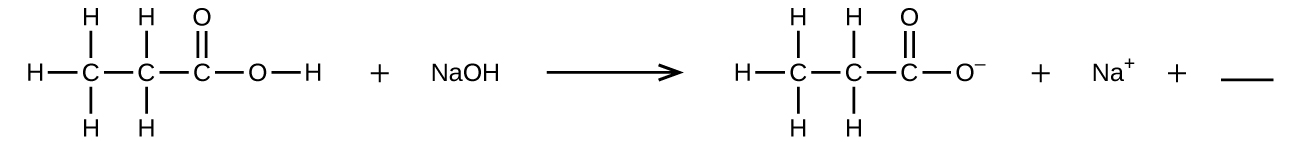
- Fill in the bare with a single chemical formula for a covalent compound that will remainder the equation:
- Aqueous hydrogen fluoride (hydrofluoric acrid) is used to compose glass and to analyze minerals for their silicon content. Hydrogen fluoride volition also react with sand (silicon dioxide).
(a) Write an equation for the reaction of solid silicon dioxide with hydrofluoric acid to yield gaseous silicon tetrafluoride and liquid water.
(b) The mineral fluorite (calcium fluoride) occurs extensively in Illinois. Solid calcium fluoride can as well be prepared by the reaction of aqueous solutions of calcium chloride and sodium fluoride, yielding aqueous sodium chloride as the other product. Write complete and cyberspace ionic equations for this reaction.
- A novel process for obtaining magnesium from sea water involves several reactions. Write a counterbalanced chemical equation for each step of the process.
(a) The outset pace is the decomposition of solid calcium carbonate from seashells to form solid calcium oxide and gaseous carbon dioxide.
(b) The second step is the germination of solid calcium hydroxide every bit the only product from the reaction of the solid calcium oxide with liquid water.
(c) Solid calcium hydroxide is then added to the seawater, reacting with dissolved magnesium chloride to yield solid magnesium hydroxide and aqueous calcium chloride.
(d) The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water.
(e) Finally, the magnesium chloride is melted and electrolyzed to yield liquid magnesium metal and diatomic chlorine gas.
- From the balanced molecular equations, write the complete ionic and net ionic equations for the following:
(a) [latex]\text{K}_2 \text{C}_2 \text{O}_4(aq) + \text{Ba(OH)}_2(aq) \longrightarrow 2\text{KOH}(aq) + \text{BaC}_2 \text{O}_2(due south)[/latex]
(b) [latex]{\text{Atomic number 82(NO}_3)}_2(aq) + \text{H}_2 \text{And then}_4(aq) \longrightarrow \text{PbSO}_4(s) + 2\text{HNO}_3(aq)[/latex]
(c) [latex]\text{CaCO}_3(s) + \text{H}_2 \text{And so}_4(aq) \longrightarrow \text{CaSO}_4(s) + \text{CO}_2(thousand) + \text{H}_2\text{O}(l)[/latex]
Glossary
- counterbalanced equation
- chemic equation with equal numbers of atoms for each chemical element in the reactant and product
- chemic equation
- symbolic representation of a chemic reaction
- coefficient
- number placed in forepart of symbols or formulas in a chemical equation to indicate their relative corporeality
- complete ionic equation
- chemic equation in which all dissolved ionic reactants and products, including spectator ions, are explicitly represented past formulas for their dissociated ions
- molecular equation
- chemic equation in which all reactants and products are represented as neutral substances
- net ionic equation
- chemic equation in which only those dissolved ionic reactants and products that undergo a chemical or physical change are represented (excludes spectator ions)
- product
- substance formed by a chemical or physical change; shown on the correct side of the pointer in a chemic equation
- reactant
- substance undergoing a chemical or concrete change; shown on the left side of the pointer in a chemical equation
- spectator ion
- ion that does not undergo a chemical or physical modify during a reaction, but its presence is required to maintain charge neutrality
Solutions
Answers to Chemical science End of Chapter Exercises
1. An equation is balanced when the same number of each chemical element is represented on the reactant and product sides. Equations must be balanced to accurately reflect the law of conservation of thing.
three.
(a) [latex]\text{PCl}_5(s) + \text{H}_2 \text{O}(l) \longrightarrow \text{POCl}_3(l) + two\text{HCl}(aq)[/latex];
(b) [latex]3\text{Cu}(s) + 8\text{HNO}_3(aq) \longrightarrow three\text{Cu(NO}_3)_2(aq) + four\text{H}_2 \text{O}(50) + 2\text{NO}(one thousand)[/latex];
(c) [latex]\text{H}_2(thou) + \text{I}_2(southward) \longrightarrow ii\text{HI}(s)[/latex];
(d) [latex]four\text{Fe}(s) + 3\text{O}_2(g) \longrightarrow 2\text{Atomic number 26}_2 \text{O}_3(s)[/latex];
(e) [latex]2\text{Na}(s) + ii\text{H}_2 \text{O}(50) \longrightarrow two\text{NaOH}(aq) + \text{H}_2(g)[/latex];
(f) [latex]\text{(NH}_4)_2 \text{Cr}_2\text{2O}_7(south) \longrightarrow \text{Cr}_2\text{O}_3(s) + \text{Northward}_2(g) + 4\text{H}_2 \text{O}(fifty)[/latex];
(g) [latex]\text{P}_4(southward) + 6\text{Cl}_2(k) \longrightarrow 4\text{PCl}_3(l)[/latex];
(h) [latex]\text{PtCl}_4(s) \longrightarrow \text{Pt}(s) + 2\text{Cl}_2(grand)[/latex];
5.
(a) [latex]\text{CaCO}_3(s) \longrightarrow \text{CaO}(s) + \text{CO}_2(one thousand)[/latex];
(b) [latex]2\text{C}_4 \text{H}_{ten}(yard) + xiii \text{O}_2(g) \longrightarrow 8\text{CO}_2(g) + ten\text{H}_2\text{O}(chiliad)[/latex];
(c) [latex]\text{MgCl}_{2}(aq) + 2 \text{NaOH}(aq) \longrightarrow \text{Mg(OH)}_2(s) + 2 \text{NaCl}(aq)[/latex];
(d) [latex]2\text{H}_2 \text{O}(one thousand) + 2 \text{Na}(s) \longrightarrow 2\text{NaOH}(s) + \text{H}_2(g)[/latex];
7.
(a) [latex]\text{Ba(NO}_3)_2[/latex] , [latex]\text{KClO}_3[/latex];
(b) [latex]2 \text{KClO}_3(s) \longrightarrow 2 \text{KCl}(s) + 3\text{O}_2(m)[/latex] ;
(c) [latex]two \text{Ba(NO}_3)_2(southward) \longrightarrow 2\text{BaO}(s) + 2\text{Northward}_2(one thousand) + 5\text{O}_2(g)[/latex] ;
(d) [latex]ii \text{Mg}(south) + \text{O}_2(g) \longrightarrow 2 \text{MgO}(south)[/latex]; [latex]4\text{Al}(southward) + three\text{O}_2(g) \longrightarrow 2\text{Al}_2 \text{O}_3(g)[/latex];[latex]4\text{Atomic number 26}(s) + 3\text{O}_2(g) \longrightarrow 2\text{Fe}_2 \text{O}_3(s)[/latex];
nine.
(a) [latex]4\text{HF}(aq) + \text{SiO}_2(s) \longrightarrow \text{SiF}_4(chiliad) + two\text{H}_2 \text{O}(fifty)[/latex];
(b) complete ionic equation: [latex]ii\text{Na}^{+}(aq) + 2\text{F}^{-}(aq) + \text{Ca}^{2+}(aq) + ii\text{Cl}^{-}(aq) \longrightarrow \text{CaF}_2(s) + two\text{Na}^{+}(aq) + 2\text{Cl}^{-}(aq)[/latex]
internet ionic equation: [latex]2\text{F}^{-}(aq) + \text{Ca}^{ii+}(aq) \longrightarrow \text{CaF}_2(due south)[/latex]
11.
(a)
[latex]2\text{K}^{+}(aq) + {\text{C}_2 \text{O}_4}^{two-}(aq) + \text{Ba}^{ii+}(aq) + two\text{OH}^{-}(aq) \longrightarrow 2\text{Yard}^{+}(aq) + 2\text{OH}^{-}(aq) + \text{BaC}_2 \text{O}_4(s) \;\text{(complete)}[/latex]
[latex]\text{Ba}^{ii+}(aq) + {\text{C}_2 {\text{O}_4}}^{2-}(aq) \longrightarrow \text{BaC}_2 \text{O}_4(s) \;(\text{net})[/latex]
(b)
[latex]\text{Atomic number 82}^{2+}(aq) + 2{\text{NO}_3}^{-}(aq) + 2\text{H}^{+}(aq) + {\text{SO}_4}^{2-}(aq) \longrightarrow \text{PbSO}_4(south) + 2\text{H}^{+}(aq) + 2{\text{NO}_3}^{-}(aq) \;\text{(consummate)}[/latex]
[latex]\text{Pb}^{2+}(aq) + {\text{SO}_4}^{2-}(aq) \longrightarrow \text{PbSO}_4(due south) \;\text{(cyberspace)}[/latex]
(c)
[latex]\text{CaCO}_3(south) + 2\text{H}^{+}(aq) + {\text{SO}_4}^{ii-}(aq) \longrightarrow \text{CaSO}_4(s) + \text{CO}_2(g) + \text{H}_2 \text{O}(fifty) \;(\text{consummate})[/latex]
[latex]\text{CaCO}_3(s) + 2\text{H}^{+}(aq) + {\text{SO}_4}^{2-}(aq) \longrightarrow \text{CaSO}_4(southward) + \text{CO}_2(g) + \text{H}_2 \text{O}(l) \;(\text{cyberspace})[/latex]
Source: https://opentextbc.ca/chemistry/chapter/4-1-writing-and-balancing-chemical-equations/
0 Response to "what mass of potassium chlorate is required to supply the proper amount of oxygen"
Postar um comentário